A Guide to Using ePRO in Clinical Trials and Patient Care
by Barbara Maley | May 20, 2021 | Data Management | comments

COVID has drastically changed everything in life — including the way we work and the way that we interact with healthcare professionals. Clinical research is one industry that has been affected the most. Just like physicians, face-to-face interaction is limited or even eliminated in some cases. Electronic patient-reported outcome (ePRO) is no longer an option – it’s the standard as a response to these changes.
In addition to responding to the pandemic, clinical trials were already transitioning to an electronic means of collecting patient data. Electronic data collection provides more accurate results, saves time when analyzing those results, and saves time and money – especially when it comes to clinical trial data management.
In this article, we will discuss:
- What is ePRO and how can it provide patient data and measure outcomes?
- Why should you consider using ePRO?
- Common methods of ePRO data collection
- How ePRO can play into your clinical trials
Our goal with this guide is to equip your team with the deep perspective that helps you conduct and manage the data within your clinical trial.
What is ePRO?
Electronic patient-reported outcomes (ePRO) in a clinical trial allow participants to answer questions and report their health through smartphones, tablets, or laptops. The clinical trial design is focused on patient experience and many clinical trials today include patient-reported outcomes. Combining patient reporting and technology can have marked increases in patient satisfaction and data accuracy.
ePRO can provide patient data and measure their outcomes in a variety of ways, including:
-
Health-Related Quality of Life: With ePRO, you can assess individuals both with and without health conditions. Most often used for patients with chronic illnesses, the data collected helps researchers, clinicians, and others to compare groups with and without a condition and estimate population norms. Because the measurement is not disease or condition-specific, you can use this data to calculate quality-adjusted life years or compare the information to current population norms.
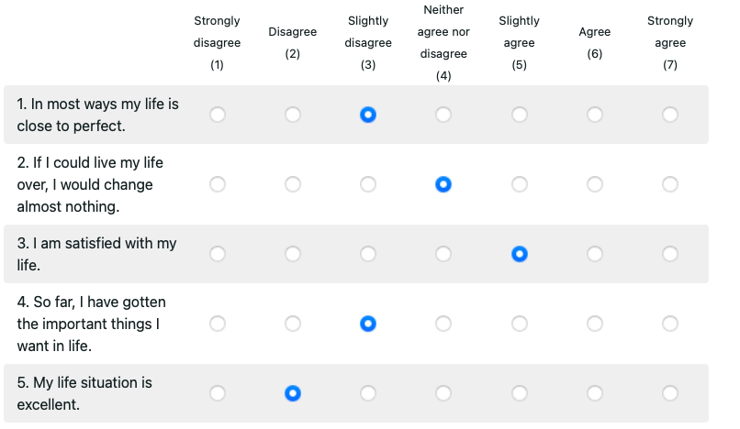
Satisfaction with Life Scale (SWLS)
- Functional Status: These measurements capture the patient’s ability to perform activities of daily life, from basic to advanced – and vary widely in quality. Could include physical, cognitive, or sexual functions.

PROMIS Physical Function
- Symptoms and symptom burden: Collecting data on symptoms such as fatigue and pain intensity are essential collection data items for ePROs. Because symptoms are typically negative, their presence and intensity are best assessed through a patient report. ePROs can be customized to use scales to measure the severity, impact, and symptom burden impacts.
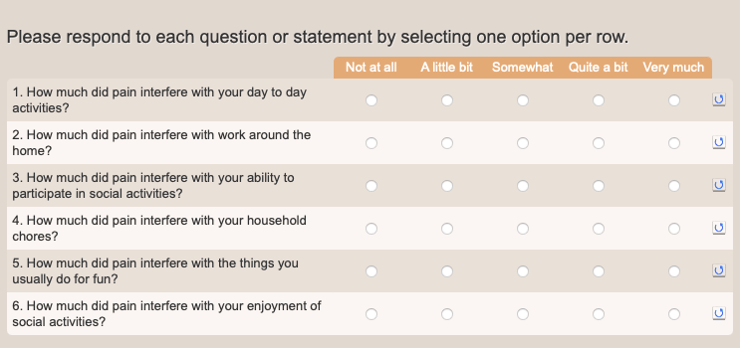
PROMIS Pain Interference
- Health risk assessments (HRAs): ePROs used to collect risk factors allow practitioners to identify areas to reduce risk and prompt healthy alternatives for their patients. This information helps identify risk and monitors health behavior over time, allowing patients to receive better quality of care and a better health care experience overall.

- Patient experience, or the overall patient rating of their healthcare: Recognizing patient preferences and values helps health care professionals improve the quality of health care and enhance healthcare expenses. When health care professionals can tailor treatments based on informed decisions, their patients have a greater sense of involvement in their care, and the result is a better experience of care.

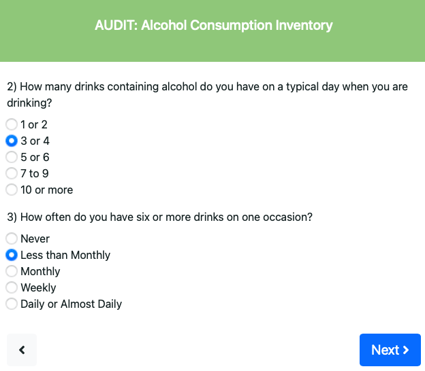
Example of AUDIT questions 1,2 and 3
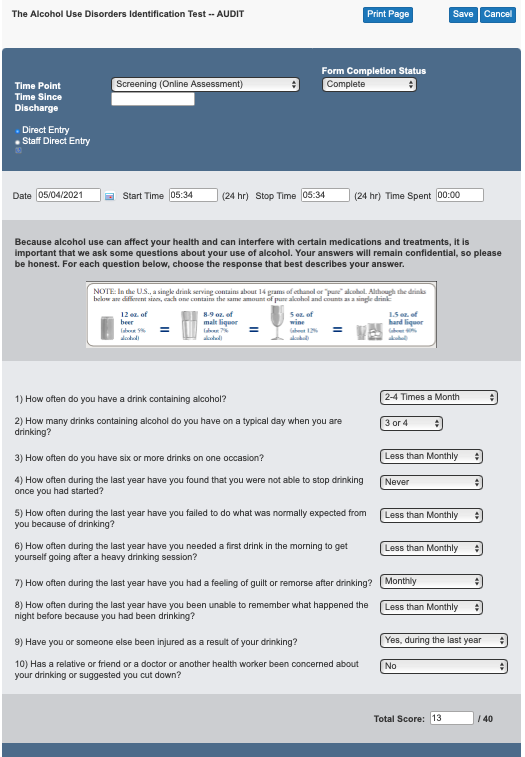
Example of AUDIT question summary
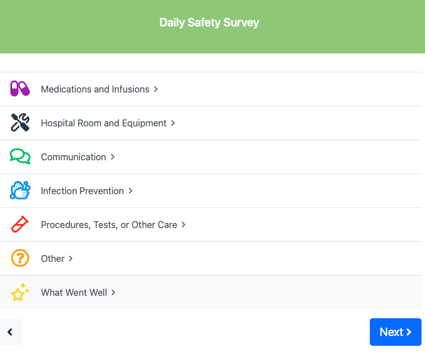
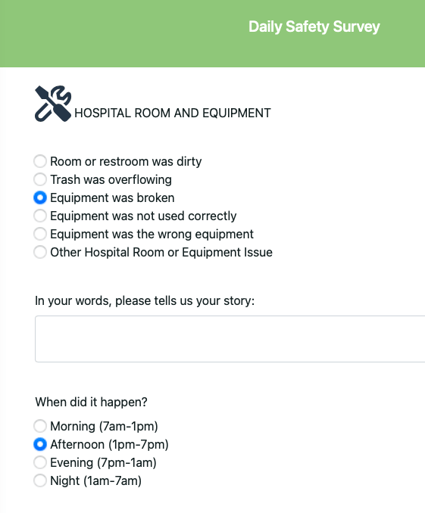
Daily Safety Survey
Why Should You Consider Using ePRO?
It’s essential to collect as much relevant data as possible on the patient experience and how well treatment is working during a clinical trial. One of the most effective and accurate tools to do this is through electronic patient-reported outcomes (ePRO).
There are really 4 major advantages to using ePRO:
- Create a better experience for patients – Patients can provide data when they cannot come during an appointment that may be difficult to schedule. This provides a more favorable impression of a patient’s involvement in a clinical trial.
- Increase efficiency for participants and researchers – ePRO shortens the timeline of gathering outcomes from patients and allowing research teams to collect and analyze the data immediately.
- Real-world data – Advances in technology such as smartwatches and Fitbit technologies allow for monitoring without a trial-provided device. This data can be transmitted to research teams for analysis, and the patient doesn’t have to change anything about their daily activities.
Cost reduction – Using ePRO saves costs on devices, duplicated data, and data collection. With these savings, it alternatively reduces cost on the number of resources and employees needed to collect and analyze the data.
What are the different data collection methods for ePRO?
Once you’ve decided the feedback you want from your patients, you can decide on the best format to capture the information. There are four basic types of ePRO collection methods. Each collection type has its own merits and disadvantages. When you are planning to incorporate ePROs, you need to consider the collection method and if it will work for your organization. You may find that using more than one collection method advantageous, depending on the patient population and intended use.
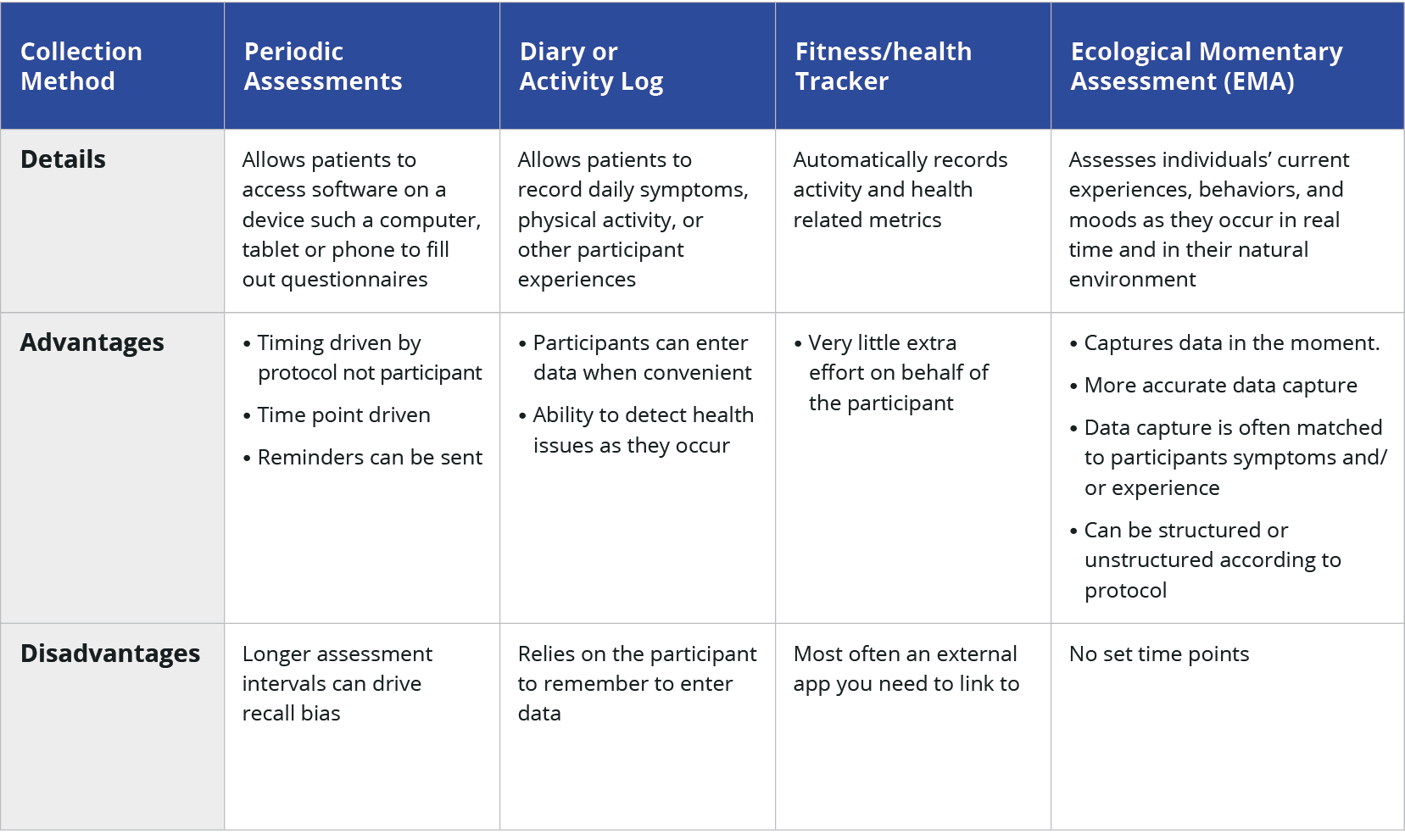
ePRO is all browser-based
The trend that computers, tablets, and phones can all support browsers has made it easier to reach users in a variety of ways improving the online data collection experience.
All formats available offer patients the flexibility to use devices provided by the trial or an app installed on their device. The familiarity with a device the patient is already using and creating a collection method that is easy-to-use increases patient satisfaction and saves you time and money to collect, analyze, and disseminate the data results. The best ePRO applications look like phone apps and are easy to use and navigate.
Smartphone-based ePRO solutions can either be user-driven, like a headache diary or activity log, or they can be system-generated where the user will receive a message that it is time to share some data. The best solutions are short assessments that take only a few minutes to complete.
Now that more people are using fitness trackers, it provides the opportunity to collect information without any intervention with the participant. These can provide insight into sleep and movement patterns, both of which can give insight into a patient’s wellbeing.
How to Use ePRO for Clinical Research
ePROs gather a wide range of measurements to cover a patient’s health status. These methods record the patient’s physical status and record the mental and social well-being of the patient. Research has shown that non-clinical factors identified when using ePROs play a vital role in a patient’s overall health. Identifying clinically significant issues increases the ability of health and research providers to address issues and proactively work to preserve patient health.
There is also evidence that the real-time data collection of ePROs plays a role in improving outcomes.
A recent clinical trial showed that the survival interval of cancer patients was increased by 5 months when using ePROs. This is partly due to staff’s ability to respond quickly to potential issues and avoid consequences due to those issues.
Key Consideration: Technology Integration
If your study population is able to use systems to manage their data, they will likely expect an easy-to-use solution to interact with your study. Some of your participants may prefer to use the computer, but many will prefer the convenience of a smartphone data collection process. Make sure that the platform that you are considering has the built-in toolset to allow you to build and deploy ePRO simply and will allow your users to interact in the way that works best for them. Your system should also have the ability to integrate to a third-party application if that is required by your study protocol.
Deciding What ePRO Is Right For You
COVID has forced health care providers and clinicians to find new and innovative ways of tracking patient data and providing health care. This change over to a digital landscape can save you time, money, and resources. But it also creates the unique challenge of finding the right system for your specific needs.
Learn how you can simplify your clinical research data collection and integrate that data directly with the QuesGen electronic data capture system. Explore ePRO today and let us know if we can answer any questions!

